
Many pharmacological drugs are enzyme inhibitors. Because enzymes catalyze most part of chemical reactions in living organisms, the enzyme inhibitors play an important role in the development of different sciences (biochemistry, physiology, pharmacy, agriculture, ecology) as well as the technologies (production of pharmaceutical drugs, insecticides, pesticides, chemical weapons, etc.). The suppression of the activity is the result of the binding of inhibitor to the enzyme molecule that arrests catalytic reaction. Definition, classification, and main propertiesĮnzymes are different chemical compounds that are combined into a group because of their only feature-they can suppress enzyme activity. Taking into account this information about enzymes in this chapter, we consider contemporary knowledge about enzyme inhibitors and activators.Ģ.1. Therefore, they will accelerate enzymatic reaction. On the other hand, the binding of enzyme activators may lead to the creation of more profitable conformers that can be more effective in carrying out definite steps of the reaction.

By this way, inhibitors stop enzymatic reaction. In the context of described hypothesis, enzyme inhibitors being bound to the enzyme “freeze” it in definite conformation that makes impossible selection of conformers participating in numerous steps of enzymatic reaction. This hypothesis is supported by unsuccessful attempts to create catalytically effective low molecular enzymes having needed active site (molecular mass should be higher than 10,000 Da) or enzyme with correct active site but with restricted conformational mobility (antibodies with needed active site, so-called abzymes ).
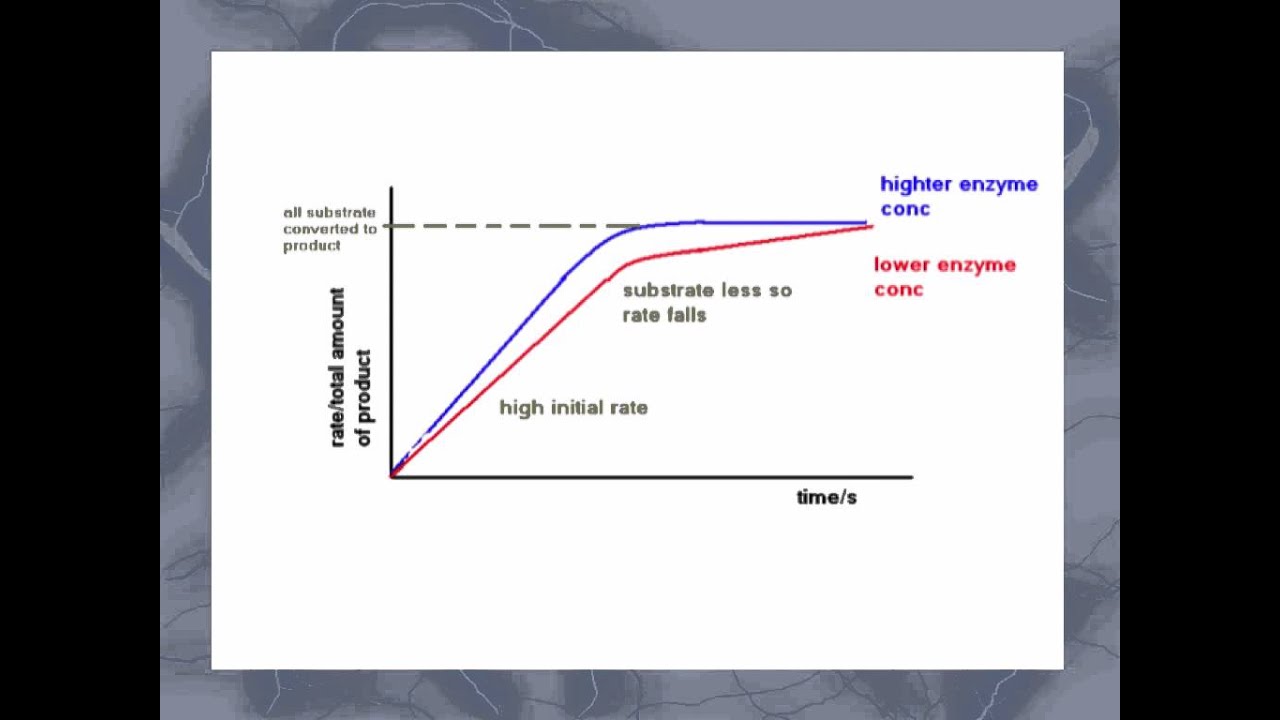

Along the reaction way, a conformer is picked out, the structure of which can stabilize definite intermediate that makes a reaction more thermodynamically profitable.

Multiple conformers of enzymes with close values of free energy preexist in the solution simultaneously. According to contemporary hypothesis, high conformational mobility of the enzymes allows them to adopt their active sites to substrate(s) and intermediates of the reaction in the best way. Because each step of enzymatic reaction has a value of activation energy significantly lower than the value of activation energy for the same chemical reaction, enzymes can increase a rate of reaction 10 6–10 18 folds. Enzymes are able to accelerate chemical reaction dividing it into separate steps. Enzymes (E) is a group of biologically active polymers (mainly proteins) that catalyze almost all metabolic reactions in all living organisms.


 0 kommentar(er)
0 kommentar(er)
